Details of the Drug
General Information of Drug (ID: DM4CDIB)
| Drug Name |
L-655708
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
130477-52-0; CHEMBL52030; NCGC00025115-02; Ethyl (S)-11,12,13,13a-Tetrahydro-7-methoxy-9-oxo-9H-imidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylate; Lopac0_000700; SCHEMBL5533646; YKYOQIXTECBVBB-AWEZNQCLSA-N; MolPort-003-983-605; HMS3262K21; ZINC2584032; Tox21_500700; MFCD02684528; BN0287; BDBM50067424; AKOS024456530; FG-8094; API0007742; LP00700; CCG-204785; CS-4943; NCGC00261385-01; NCGC00025115-04; NCGC00025115-03; HY-14426; KB-78047; EU-0100700; L-655,708, >
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
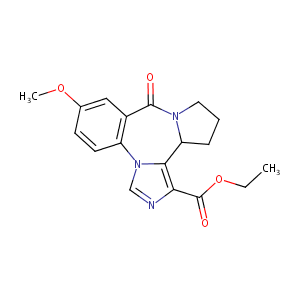 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 341.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
